

Web the molecular orbital diagram has molecular orbital energy level at centre and is surrounded by atomic orbital energy level. (c) with reference to the molecular orbitals (mos) theory: (atomic number of ne = 10). The same method can be applied to other diatomic molecules. Neon atom has 10 electrons and its electronic configuration is.when molecule is considered, it has two neon atoms and. Source: Check Detailsĭraw out the molecular orbital diagram for ne2, starting with the 2s atomic orbitals and label each molecular orbital with the appropriate notation as done in class. Web with the help of molecular orbital diagram show that ne2 cannot exist as stable molecule. It shows electrons in both bonding and anti. I have been taught that the mo diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. Draw out the molecular orbital diagram for ne2, starting with the 2s atomic orbitals and label each molecular orbital with the appropriate notation as done in class. (i) sketch the mo diagram of ne2, justify and discuss its energy level diagram. Web there are two mo diagrams you need to memorize for diatoms (n2, o2, ne2, etc).one is for the elements up to nitrogen.
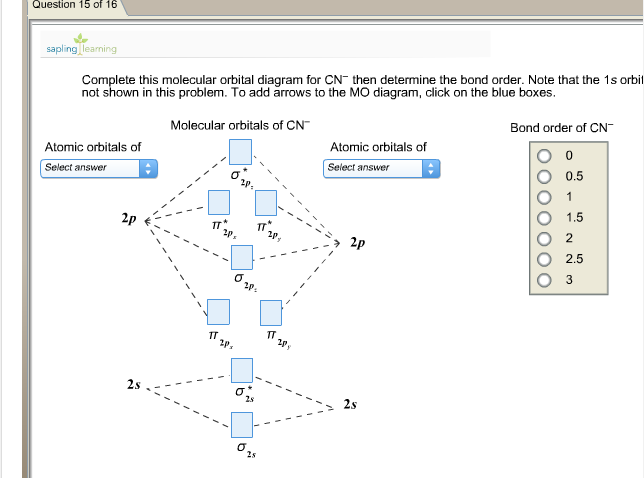
This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown. Web a diatomic molecular orbital diagram helps us deduce the magnetic properties of the molecule, changes during ionization, bond order, and the number of bonds formed.
#MOLECULAR ORBITAL THEORY DIAGRAM FREE#
Ne2 Molecular Orbital Diagram Free Wiring Diagram from


 0 kommentar(er)
0 kommentar(er)
